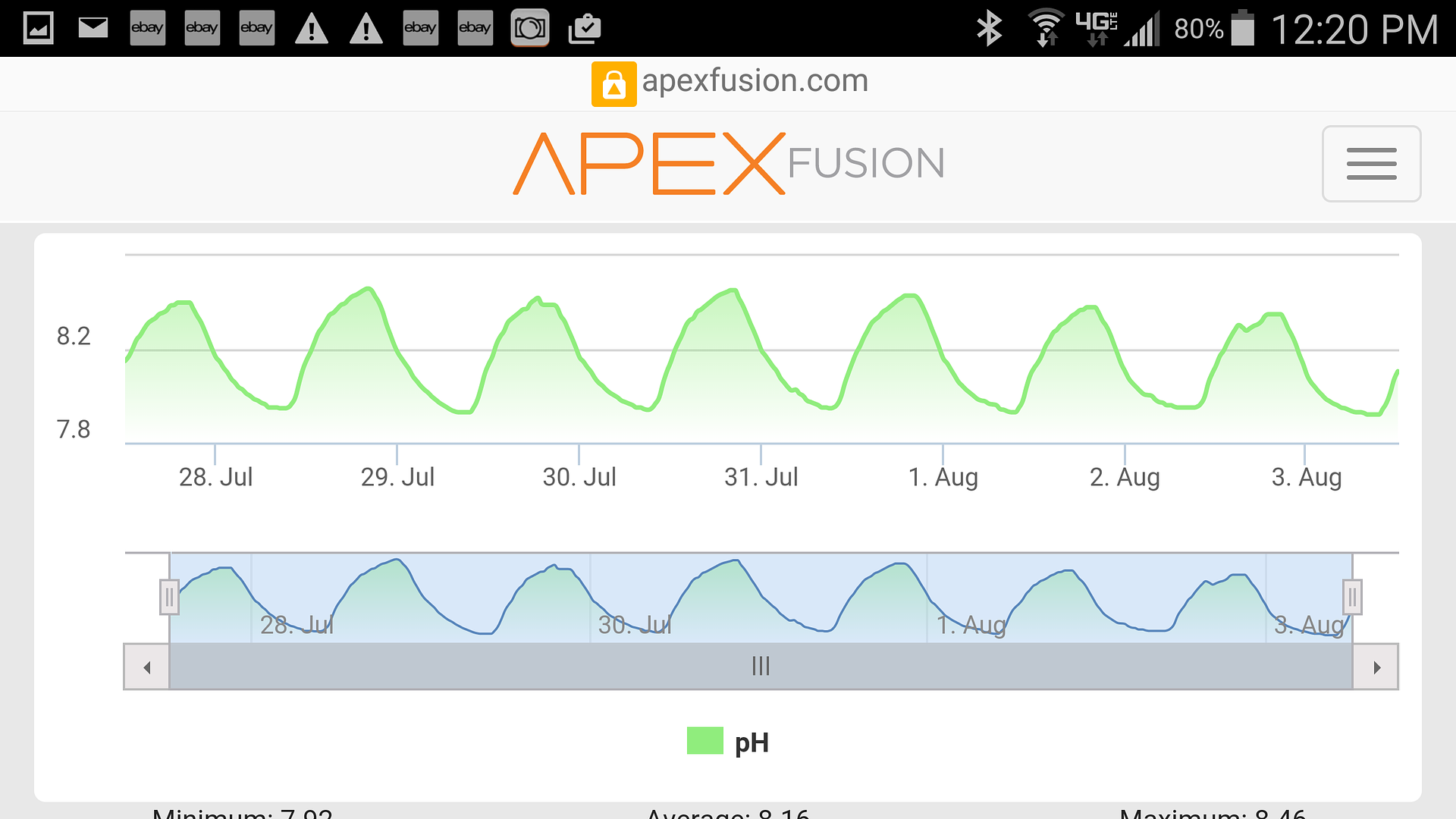
I'm starting to question the advice you're getting from that fish store. If they are telling you to chase a PH number with additives then I would suggest finding a different source of advice.
PH in seawater is a function of alk and Co2. If your alk is in the normal range (say 8-12) then your PH WILL be in the normal range (say 7.8-8.4) unless there is a lot of Co2 driving the PH down. PH is not the number to be paying attention to, alk is. If the alk is normal and the PH low, then you need to look at Co2. Co2 will come from elevated Co2 in the air around the tank, for example of the house is closed up all the time with AC keeping it cool allowing Co2 to build up, OR it could be excess Co2 from your Ca reactor.
Next thought... "white stuff on the tip of the rocks" This easily could be precipitation caused by having too much Ca/alk or having good levels and then adding too much of a supplement. Considering that you just added a supplement to water that seemed to be in the normal Ca/alk ranges, and now you are seeing white stuff, you may well be edging on a serious precipitation event aka snow storm. (snowstorm meaning massive precipation, suddenly the Ca and all start sticking together in mass and you literally see white stuff falling out of solution which looks like it's snowing in your tank.)
Now really jumping......... Maybe your Mg is low making it hard to keep Ca and alk in normal range. With that, you need to keep the Ca reactor cranked way up causing too much Co2 going into the water, which in turn causes the PH to be driven down falsely suggesting that something else is low.
Hmm, Dong (dz6t) you're the chemist here - am I heading in the right direction? Please explain in more educated terms